Radical Tool in Nature's Chemistry Toolbox Depicted
Researchers at DBB have determined the structure of one of nature's sharpest chemical tools, a protein radical. The results help us understand how nature can carry out very challenging chemical reactions and can ultimately be used in both medicine and green chemistry applications.
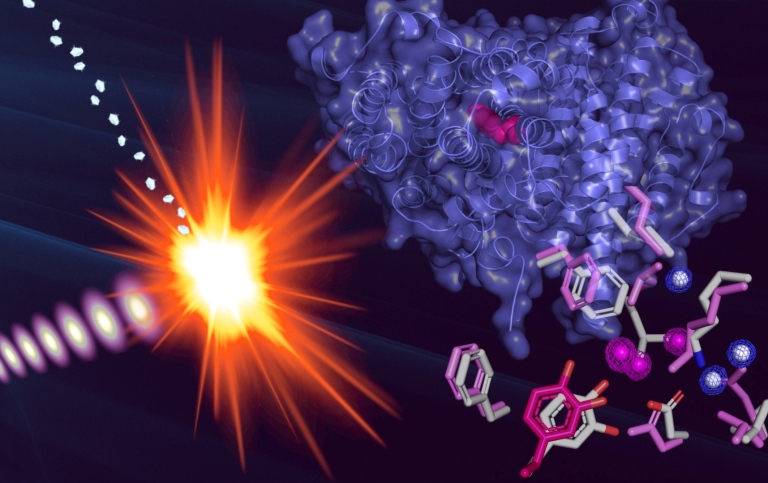
A radical is a chemical compound with an unpaired electron, making it highly reactive. Free radicals can cause damage to cells, but nature has also found ways to tame them and harness the properties of radicals to perform particularly difficult chemical reactions. For example the protein ribonucleotide reductase, which manufactures the building blocks of DNA, uses a radical to carry out this essential reaction.
To be able to perform this chemistry, a very reactive molecule is needed, but just like for a sharp knife, the radical must be protected and controlled so that it can be used without causing damage to the cell. The fact that the radical is so reactive also makes it very difficult to study structurally because it is immediately destroyed in the process. This was overcome by utilizing extremely short and intense X-ray laser pulses, allowing the data to be collected before radiation damage has time to occur. The project, led by researchers at DBB was carried out at the LCLS (Linac Coherent Light Source) in Stanford, California, in collaboration with researchers from France and the USA.
By comparing the structure of the protein with and without the radical, the researchers can explore how the protein can protect the radical and also mobilize it for use. In addition to better understanding of nature's chemistry, the results are relevant for both bio-inspired chemical synthesis and medicine. Ribonucleotide reductase is essential for cell division, and if it can be stopped, cell growth is also halted. RNR is thus a target for treatment of cancer and the development of new antibiotics.
Read the article ”Structure of a ribonucleotide reductase R2 protein radical” in Science
The research was funded by the Knut och Alice Wallenbergs stiftelse, ERC (Europeiska forskningsrådet) and Vetenskapsrådet.
Last updated: October 12, 2023
Source: DBB


