Researchers at SU and Imperial College show how Photosystem II can produce reactive oxygen species
Photosystem II (PSII) is a key enzyme in photosynthesis that produces oxygen by capturing sunlight. The reaction couples to the reduction of plastoquinone, which in turn is used for the synthesis of biomass by fixation of CO2.
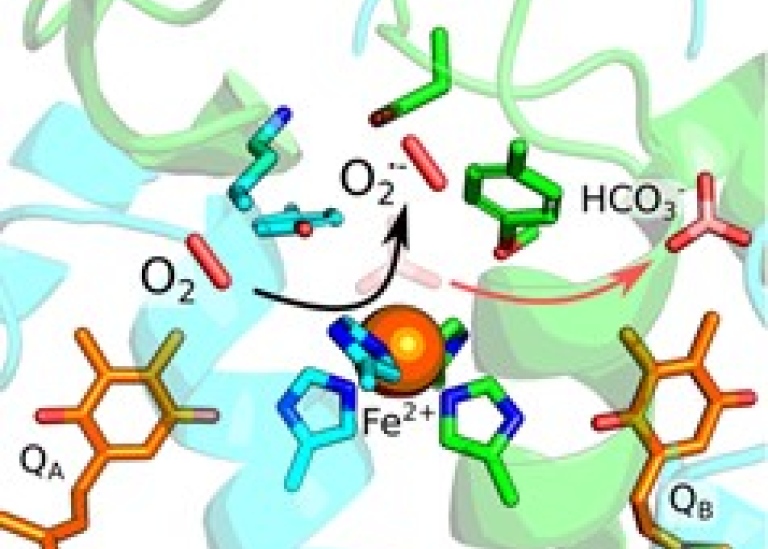
Now researchers from the Kaila lab at Stockholm University have together with the Rutherford lab at Imperial College London demonstrated how PSII can also produce a reactive oxygen species called superoxide (O2•-). These highly reactive molecules can be harmful for the cell, but they also have important functional roles in signaling and regulation.
The superoxide production is catalyzed by the non-heme Fe2+ site in PSII via electron transfer from one of the plastoquinone sites (QA). The process was found to be regulated by inorganic bicarbonate (HCO3-), which normally binds to the Fe2+ site, and thus has a protective role. The researchers further showed that in the absence of bicarbonate, oxygen binds to the Fe2+ and steals an electron from the plastoquinone site, forming superoxide. These unexpected findings have important implications for understanding the cross-talk between the regulation of PSII and CO2 fixation.
The study was recently published in PNAS and funded by Knut and Alice Wallenberg (KAW) foundation, BBSRC, and the TU Munich-Imperial College strategic partnership Global Incentive Fund.
Fantuzzi A, Allgöwer F, Baker H, McGuire G, Teh WK, Gamiz-Hernandez AP, Kaila VRI, Rutherford AW. Bicarbonate-controlled reduction of oxygen by the QA semiquinone in Photosystem II in membranes.
Proc Natl Acad Sci USA. 2022;119(6):e2116063119. doi: 10.1073/pnas.2116063119
PNAS article: https://www.pnas.org/content/119/6/e2116063119
Ville Kaila Lab: https://villekaila.com/
Bill Rutherford Lab: https://www.imperial.ac.uk/people/a.rutherford
Last updated: March 15, 2022
Source: Department of Biochemistry and Biophysics

