Scientists show how a salt transporter linked to hypertension and diabetes works
NHA2 is a protein spanning the membrane in kidney and beta-cells that absorbs salt and its activity is linked with hypertension and diabetes in humans. The transporter-protein exchangers the movement of sodium ions across cell membranes with the movement of protons. Researchers at Stockholm University have now determined what the protein looks like and how the protein adapts to the membrane. These findings shed new light into this important biological mechanism, and provide a basis for structural-based drug discovery.
The regulation of sodium levels and intracellular pH is a fundamental process in most living organisms. Na+/H+ exchangers are found in every cell and couple the movement of protons with that of sodium ions to fine-tune the cells internal pH, sodium levels and cell volume. Na+/H+ exchanger dysfunction has been linked to many diseases such as cancer, hypertension, heart failure, diabetes, and epilepsy. In particular, NHA2 is found in the kidney and b-cells and was recently identified as the long-sort after salt transporter linked to hypertension and diabetes in humans 1,2. Consequently, inhibition or modulation of NHA2 activity by drugs could provide a new therapeutic intervention against these diseases. Unfortunately, very little was known about how NHA2 works. Here, by combining electrophysiology, biochemistry, MD simulations, native mass-spectroscopy and cryo EM microscopy a team lead by David Drew at Stockholm University has been able to show what the protein looks like, and how the protein rearranges itself in the presence of a specific lipid to become more active. These findings reveal a unique adaption of a salt-transporter to a membrane environment with important physiological ramifications.
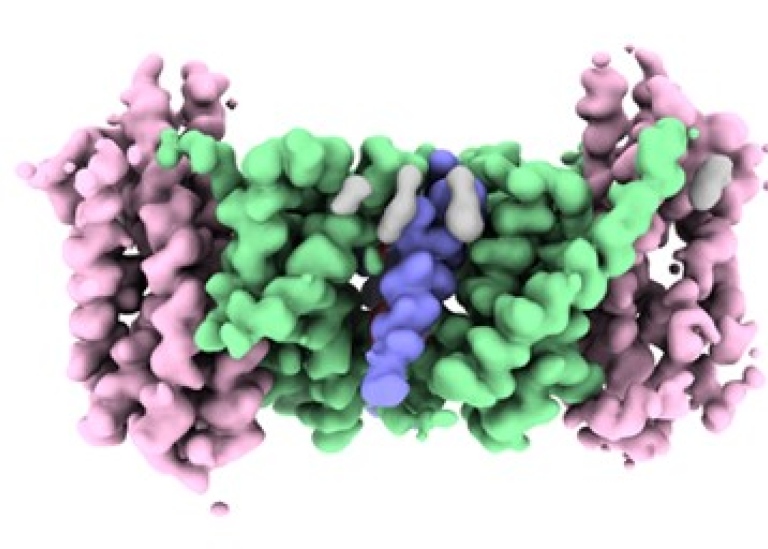
The work was a collaboration between Stockholm University, Karolinska Institiutet, Oxford University, Kyoto University, Arizona State University and was funded by Vetenskapsrådet and the European Research Council (ERC) Consolidator grant EXCHANGE to David Drew at SU. The article "Structure, mechanism and lipid-mediated remodeling of the mammalian Na+/H+ exchanger NHA2” is published in the scientific journal Nature Structural & Molecular Biology on February 16th 2022.
DOI: https://doi.org/10.1038/s41594-022-00738-2
https://www.nature.com/articles/s41594-022-00738-2
1. N. Engl. J. Med. 318, 146–150 (1988).
2 N. Engl. J. Med. 302, 772–776 (1980).
Last updated: March 16, 2022
Source: Department of Biochemistry and Biophysics

