Quantum Chemistry Meets Quantum Electrodynamics: Molecule-Cavity Interaction Simulation
Simulating interactions between molecules coupled to an optical cavity is possible with a new model that combines quantum electrodynamics and a widely used formalism from quantum chemistry. Markus Kowalewski is associate Professor at Fysikum and is responsible for the research group Molecular Quantum Dynamics and Spectroscopy (TQDSpec).
The use of tightly confined light modes to manipulate atoms and molecules has become a popular method for exploring new states of matter. Interactions between confined light modes in nano-cavities and molecules can give rise to so-called "molecular polaritons", which significantly influence chemical reactions and molecular properties. The experimentally observed effects are often discussed in a phenomenological way, and the understanding of the underlying microscopic and macroscopic physical mechanisms is still incomplete. Therefore, accurate theoretical models that account for intermolecular interactions to describe ensembles of molecules are essential to understand the mechanisms that govern polaritonic chemistry. We have developed an ab-initio quantum mechanical approach to study molecular polaritons.
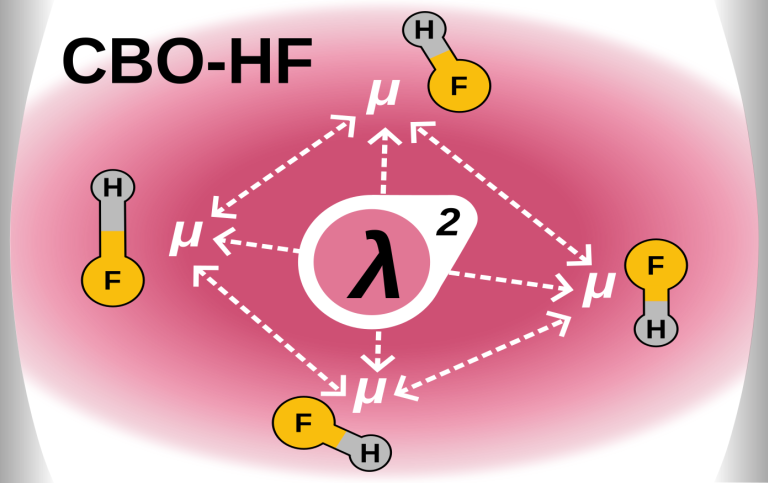
The Hartree-Fock ansatz is a well-known formalism in quantum chemistry to approximate the many-electron wave function and energy of a molecular system in its ground state. We reformulate this ansatz in the framework of the cavity Born-Oppenheimer approximation, which provides a non-perturbative, self-consistent description of molecular ensembles strongly coupled to an optical cavity. We call the resulting method cavity Born-Oppenheimer Hartree-Fock (CBO-HF) and use it to study the collective effects in small ensembles of diatomic hydrogen fluoride molecules in an optical cavity.
Thus we were able to identify a non-trivial interplay of global and local interactions between the molecules. This mechanism is based on the cavity mediated dipole-dipole interactions and it allows for local changes to affect the whole ensemble of molecules. This newly identified mechanism may be important in the presence of thermal fluctuations and could be a possible explanation for the modified in chemical reactivity in nano-cavities.
Besides the present findings, the developed CBO-HF approach provides a suitable numerical framework for more sophisticated electronic structure calculations and also paves the way to perform ab initio semiclassical and full quantum dynamics simulations of molecular ensembles strongly coupled to a cavity.
More information
Cavity Born–Oppenheimer Hartree–Fock Ansatz: Light–Matter Properties of Strongly Coupled Molecular Ensembles - ACS Publications
Last updated: October 4, 2023
Source: Fysikum