Coronene molecules contribute to complex chemistry in space
Carbon forms the basis of all organic chemistry and thus the building blocks of life. There is increasing evidence that amino acids and other complex organic molecules can be formed in space and spread to planets through, e.g., comet impacts. Large carbon-based molecule such as coronene could play an important role in how such organic molecules are produced in astronomical environments. Michael Gatchell has been interested in understanding the universe for as long as he can remember. Here he tells us about his research at Atomic Physics division of Fysikum and new results can change how we imagine molecules such as coronene contribute to chemistry in space.
”For as long as I can remember I have been interested in understanding how the universe works. My favorite subjects were physics, chemistry, and astronomy. When applying for university I ended up pursuing studies in astronomy at Stockholm University, earning my master’s degree there in 2011. I then starting looking for PhD programs in astronomy and physics before ending up at the Atomic Physics division at Fysikum.”
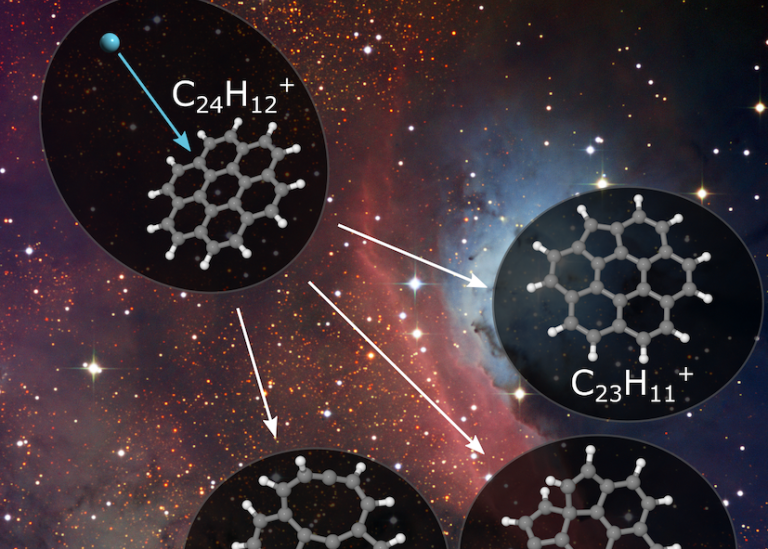
Coronene molecules consist of seven carbon rings surrounded by hydrogen atoms
“The system we have studied in our recent work is coronene, more specifically positively charged coronene ions. Coronene belongs to the category of molecules known as Polycyclic Aromatic Hydrocarbons, or PAHs for short. PAH molecules all consist of rings of carbon atoms, typically six atoms per ring, that are fused into a single, flat structure that is similar to graphene. Coronene consists of seven such carbon rings, with 24 carbon atoms in total, and its edges are capped off with hydrogen atoms that stabilize the vacant bonds.”
PAH molecules are abundant in space
“These types of molecules are so abundant that perhaps as much as 20 percent of all of the elemental carbon in the universe is believed to be locked up in PAHs. The reason for their high abundance in space is that they are very stable molecules. Many regions of space are swamped with hazardous radiation in the form of ultraviolet light, emitted by young stars, and from energetic particles, emitted as stellar winds or in supernova shockwaves, that can readily damage or destroy molecules and larger particles (such as dust grains) present amongst the stars in galaxies such as our own Milky Way. Because of this, only the most stable molecules will be able to survive in many astronomical environments.”
PAH molecules contribute to star formation in large interstellar clouds
“A result of PAH molecules being stable is that they are not particularly reactive from a chemical standpoint, that is, they do not easily form chemical bonds with other molecules that they come into contact with. PAH molecule are, nevertheless, believed to play an important role in various chemical processes that take place in space. For instance, they can help with converting free hydrogen atoms (H) into hydrogen molecules (H2), which is an important step in star formation in molecular clouds. They may also contribute to the growth of other large, carbon-based molecules in space. Carbon is, after all, the basis of all organic chemistry and there is evidence that amino acids and other complex organic molecules form in space and could then spread to planets via, e.g., impacting comets.”
Removing single carbon atoms from PAH molecules increases their reactivity
“In previous studies we have been able to drastically increase the chemical reactivity of PAH molecules by knocking out individual carbon atoms from their molecular structures. We do this by bombarding the molecules with atoms traveling at speeds of around 100 kilometers per second. When this happens, an incoming atom can directly strike one of the atoms in the molecule, knocking it out of the molecule in a process that is a lot like when two pool balls hit each other. What remains is a damaged PAH fragment that is far more reactive than an intact PAH molecule. We found that such fragments readily react with other molecules to form larger structures.”
An isolated molecule may wait for years before finding another particle to react with
“A velocity of 100 km/s may sound very fast, but it is actually pretty typical for the velocities found in stellar winds and supernova shockwaves, both of which consisting primarily of low mass atoms such as hydrogen and helium. It is therefore highly probable that processes where atoms are knocked out of PAH molecules (or other types of molecules) are common in the astronomical environments where these are present. But a remaining question has always been whether or not the reactive PAH fragments that we have been studying can survive long enough to contribute to different chemical reactions in space. Despite many interstellar environments containing a rich chemical inventory of different types of atoms and molecules, the densities in those regions are very low. An isolated molecule may thus wait for many years before finding another particle to potentially react with. However, the experiments we have done in the past to study these processes have only allowed us to follow our molecules and fragments for timescales of up to a few microseconds (millionths of a second).”

Experiments carried out using DESIREE at Fysikum
“Our new experiments were carried out using DESIREE, which is a facility at Fyskium at Stockholm University for storing and studying ions (charged atoms or molecules). The core of DESIREE consists of two storage rings that are used to store ions on long timescales (up to several hours). We achieve this by operating the interior of DESIREE at an extremely low vacuum pressure and a temperature that is only about 10 degrees above absolute zero, approximately –260 °C. The cold and nearly empty environment in DESIREE is vital for being able to store ions for any significant length of time since these are easily destroyed in collisions with any of the remaining residual gas molecules still present in the experiment.”
PAH fragments can survive in astronomical environments
“In our experiments we collied coronene ions with helium atoms in order to knock individual atoms out of the ions. We then stored the remaining fragments in DESIREE and studied how they stabilize as a function of time. The fragments that are formed when an atom is knocked out of a PAH molecule will be heated by the collisions. This extra energy could cause the fragments to be broken down shortly after they are formed. In our measurements we saw that most of the fragments were stable and that only a small fraction of them would be lost in this way. After a hundredth of a second, all of the fragment stored in DESIREE had stabilized and at times longer than this all of the fragments had cooled sufficiently so that they would survive forever if they never came into contact with another particle or were heated up by another process. We could thus show in a clear way that reactive PAH fragments can survive in astronomical environments and therefore contribute to various chemical processes that take place in space.”
Collisions between atoms and PAH molecules
During Michael’s time as a PhD student, the group he was a part of studied collisions between atoms and PAH molecules (amongst many other systems), a subject that has been a focus of his former advisor Henning Zettergren for some time. Michael defended his PhD in 2016 with a thesis covering how carbon-based molecules react when atoms are removed by collisions.

Studies of ultra-cold helium droplets at the University of Innsbruck
After finishing his PhD, he received a grant from the Swedish Research Council (Vetenskapsrådet) to travel as an international postdoc to the University of Innsbruck for three years. There Michael studied the properties of ultra-cold helium droplets and how they could be used for different experimental studies of cold clusters consisting of loosely bound complexes of atoms or molecules.
“I returned to Fyskium one year ago and received a starting grant from Vetenskapsrådet. In my current project I am developing new techniques that take advantage of helium droplets for studying how, for example, organic molecules are formed from simple building blocks in astronomical environments.”
Michael is also involved in the work surrounding DESIREE, which made these new results possible. This new work is a natural continuation of the work he did as a PhD student.
“It has been nice to achieve these results after ten years of working with the topic. Outside of work I spend most of my time with my family, including my five-year-old son and soon our second one that is due any day now. We enjoy hiking and cycling together, something we spent a lot of time doing during our years in Innsbruck.”
Further information
Article published in Nature Communications: Survival of polycyclic aromatic hydrocarbon knockout fragments in the interstellar medium
Last updated: June 29, 2023
Source: Gunilla Häggström, Communications Officer, Fysikum


