Directly observing how carbon dioxide is transformed into fuels
Researchers at Stockholm University have developed a novel approach to examine chemical reactions and have applied it to the reduction of carbon monoxide by copper, a crucial step in the conversion of carbon dioxide into fuels. The results have recently been published in the journal Angewandte Chemie.
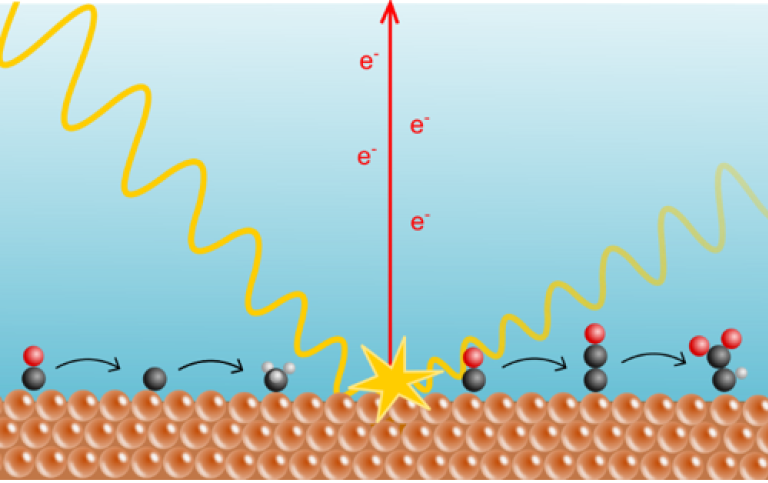
Electrical power can be used to recycle CO2 into fuels via electrochemical CO2 reduction. When we use renewable electricity for this, we can continue to use the carbon-based fuels we rely upon, while reducing our dependence on fossil fuels and addressing climate change. Copper is the most effective catalyst for the CO2 reduction reaction, but why that is and how it operates is not well understood. A team of researchers, led by Sergey Koroidov from the department of physics at Stockholm University, adapted previously used experimental methods to acquire this knowledge.
"There is an effective technique which measures electrons, generated by X-rays, to follow how the atoms on the surface transform,” Koroidov says, “However, this method is difficult to adapt to our reaction since the presence of a liquid electrolyte can inhibit our ability to measure the emitted electrons. To enable this study, we managed to engineer a new reaction setup with a sufficiently thin electrolyte layer for the electrons to pass through, but still thick enough for the chemicals to react.”
In the study, the researchers used their refined method to study the later parts of the CO2 reduction reaction, in which the starting molecule is carbon monoxide, or CO.
“CO can turn either into valuable compounds such as acetate, or into less useful products such as methane. Our study shows which pathways the carbon atoms take to produce these very different chemicals. This knowledge aids in designing new and improved catalysts, which enable us to produce more affordable fuels from renewable electricity,” says Bernadette Davies, a Ph.D. student in the chemistry department at Stockholm University.
The study was conducted by an international team of researchers, including participants from the chemistry and physics departments at Stockholm University, utilizing an instrument developed by Anders Nilsson's group in the physics department.
“The adaptation of our instrument to the study of electrochemical catalysts opens the door for a new suite of reactions that were previously inaccessible. We will continue making advancements in this area in the future,” says David Degerman, a postdoctoral researcher at Stockholm University.
More information
Scientist contact: Sergey Koroidov, sergey.koroidov@fysik.su.se
Further reading: https://onlinelibrary.wiley.com/doi/10.1002/anie.202506402
Last updated: August 19, 2025
Source: Gunilla Häggström, Communications Officer, Fysikum