Liquid-liquid phase separation in supercooled water from ultrafast heating of low-density amorphous
Recent experiments continue to find evidence for a liquid-liquid phase transition (LLPT) in supercooled water, which would unify our understanding of the anomalous properties of liquid water and amorphous ice. These experiments are challenging because the proposed LLPT occurs under extreme metastable conditions where the liquid freezes to a crystal on a very short time scale.
Here, we analyze models for the LLPT to show that coexistence of distinct
high-density and low-density liquid phases may be observed by subjecting
low-density amorphous (LDA) ice to ultrafast heating. We then describe
experiments inwhich we heat LDA ice to near the predicted critical point of the
LLPT by an ultrafast infrared laser pulse, following which we measure the
structure factor using femtosecond x-ray laser pulses. Consistent with our
predictions, we observe a LLPT occurring on a time scale < 100 ns and widely
separated from ice formation, which begins at times >1 μs.
Evidence for a liquid-liquid critical point (LLCP) has recently been reported
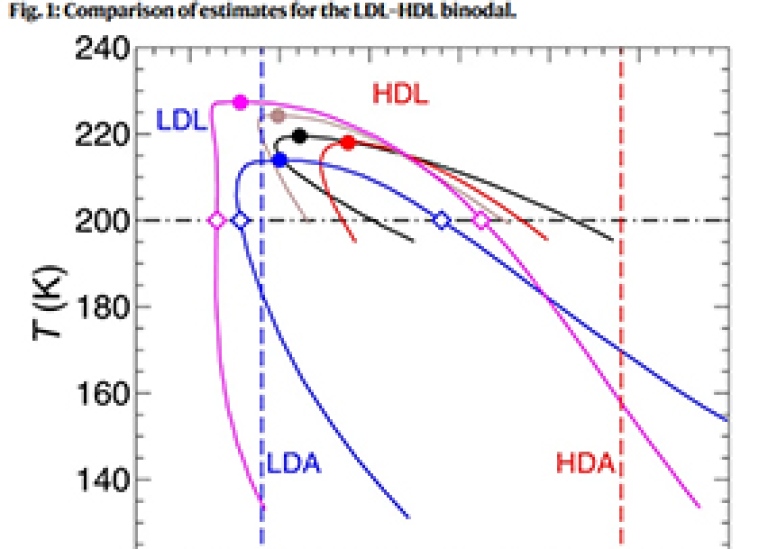
A growing body of experimental evidence supports the existence of a liquid-liquid phase transition (LLPT) in supercooled water, in which low-density liquid (LDL) and high-density liquid (HDL) phases become distinct below a critical temperature, Tc1,2,3,4,5,6,7,8. A LLPT has been experimentally observed in other systems such as phosphorous and sulfur, and evidence for a liquid-liquid critical point (LLCP) has recently been reported for the latter9,10. However, experiments testing for a LLPT in water are challenging because, unlike in phosphorous and sulfur, the proposed transition occurs under extreme metastable conditions for the liquid phase. Recent thermodynamic modeling predicts that the LLCP in water occurs at Tc ≃ 220 K and a critical pressure Pc ≃ 13–72 MPa11,12,13. Under these conditions, crystalline ice begins to form in a bulk liquid sample on a time scale of 1 to 10 μs8. As a consequence, an experiment to observe the LLPT in supercooled water must be fast: It must first produce a liquid sample that is both supercooled and under pressure, and then measure its properties, all on a sub-μs time scale before crystallization intervenes.
Hard x-ray laser pulses were used to characterize the structure
A novel solution to this challenge was recently exploited to provide direct experimental evidence of the LLPT in supercooled water8. In this “pump-probe” approach, an ultrafast infrared (IR) laser pulse was used to heat a sample of high-density amorphous ice (HDA) to a point in the phase diagram at approximately 205 K and 300 MPa where the system was in the HDL phase. Since the time required for heating was much less than the time required for sound propagation through the sample, the heating process was isochoric. After heating, the internal pressure of the sample relaxed to ambient pressure over a time scale of 10 ns to 100 μs, carrying the sample through the conditions predicted for the phase transition from HDL to the LDL phase. Hard x-ray laser pulses were used to characterize the structure of the sample as a function of time as the pressure decreased, and revealed two distinct transitions well separated in time, first from HDL to LDL, and then from LDL to ice8. Other recent pump-probe studies of amorphous ice are described in refs. 14,15.
The density of the starting material is an important control parameter
Ultrafast isochoric heating is a valuable approach for studying the LLPT in water because it can move an amorphous solid sample to conditions relevant for studying the LLCP on a time scale (fs to ps) that is much shorter than can currently be achieved when cooling a bulk sample from the liquid phase (requiring 10–100 μs or more)6. In addition, the isochoric nature of the process provides access to thermodynamic pathways that are not commonly explored in experiments, and also means that the density of the starting material (HDA ice in ref. 8) is an important control parameter that determines the pathway followed in the experiment, as illustrated in recent simulations 16,17.
The coexistence of LDL and HDL phases within a single sample
Here, we examine the consequences of applying the same ultrafast isochoric heating procedure used in ref. 8 to low-density amorphous ice (LDA). From an analysis of thermodynamic models developed by Anisimov and coworkers11,12,13, we first show that isochoric heating of LDA has the potential to move the sample to a state point much closer to the LLCP than is achieved by heating HDA. We then test this prediction in ultrafast laser heating experiments of LDA. Our experimental results are consistent with our predictions, and show that ultrafast heating of LDA provides a novel pathway to observe the rapid generation of coexistence of LDL and HDL phases within a single sample.
More information
Read more in the article published in Nature Communications
Last updated: February 2, 2023
Source: Fysikum