Mitochondrial research
Mitochondria are central to the conversion of nutrient energy into cellular energy by oxidative phosphorylation. Any chronic imbalance of mitochondrial energy homeostasis would impact cellular energy metabolism, resulting in metabolic diseases. A comprehensive quantitative assessment of mitochondrial bioenergetics is pivotal to identify defective mitochondrial mechanisms and pathways, thus to discover therapeutic drug targets to fight diseases such as obesity, diabetes and aging-related degeneration.
Therefore, our research goes beyond conventional correlation of gene products and function by directly assessing mitochondrial activity in the intact mitochondrion and cell. Mitochondrial oxygen consumption, membrane potential and reactive oxygen species production provide powerful readouts to demonstrate mitochondrial function and dysfunction (Jastroch et al. 2010, Essays in Biochemistry). We perform analyses on isolated mitochondria to identify intrinsic factors that alter mitochondrial energy transduction.
Cellular energy metabolism and consequences
We use novel sophisticated technologies (e.g. extracellular flux analyzer) to partition cellular oxygen consumption for analysis of mitochondrial efficiency in living cells. Recently, we reported on the interplay between mitochondrial dynamics and bioenergetics in pancreatic beta cells (Kabra et al. 2017, Diabetes), showing the influence of mitochondrial shape changes on insulin secretion. Our expertise is witnessed by many collaborative projects assisting colleagues to pinpoint defects in cellular energy metabolism.
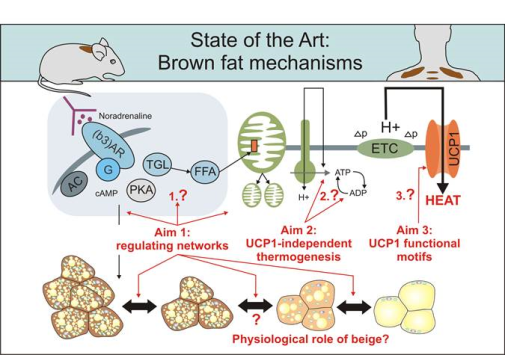
Keywords
mitochondria, bioenergetics, brown adipose tissue, thermogenesis,mitochondrial proton leak, comparative physiology
Selected publications
- Keipert S, Kutschke M, Ost M, Schwarzmayr T, van Schothorst EM, Lamp D, Brachthäuser L, Hamp I, Mazibuko SE, Hartwig S, Lehr S, Graf E, Plettenburg O, Neff F, Tschöp MH, Jastroch M. Long-Term Cold Adaptation Does Not Require FGF21 or UCP1. Cell Metab. 2017 26(2):437-446.e5.
- Gaudry MJ, Jastroch M, Treberg JR, Hofreiter M, Paijmans JLA, Starrett J, Wales N, Signore AV, Springer MS, Campbell KL. Inactivation of thermogenic UCP1 as a historical contingency in multiple placental mammal clades. Science Adv. 2017 3(7):e1602878.
- Oelkrug R, Goetze N, Exner C, Lee Y, Ganjam GK, Kutschke M, Müller S, Stöhr S, Tschöp MH, Crichton PG, Heldmaier G, Jastroch M, Meyer CW. Brown fat in a protoendothermic mammal fuels eutherian evolution. Nat Commun. 2013 4:2140.
- Jastroch M, Withers KW, Taudien S, Frappell PB, Helwig M, Fromme T, Hirschberg V, Heldmaier G, McAllan BM, Firth B.T, Burmester T, Platzer M, Klingenspor M. Marsupial uncoupling protein 1 sheds light on the evolution of mammalian nonshivering thermogenesis. Physiol Genomics 2008 17;32(2):161-9.
- Jastroch M, Wuertz S, Kloas W, Klingenspor M. Uncoupling protein 1 in fish uncovers an ancient evolutionary history of nonshivering thermogenesis. Physiol Genomics. 2005 22, 150 – 156. featured by the journal
See all publications
(Link to PubMed)





