Policy brief: Ocean acidification poses another threat to the Baltic Sea ecosystem
In the coming decades, ocean acidification is expected to become significant also in the Baltic Sea. For an already stressed ecosystem, it represents an additional pressure, and the cumulative effect of this and other environmental impacts can stress species and reduce biodiversity. Protecting the unique environment and future food production requires both significant reductions in carbon dioxide emissions and measures against eutrophication, overfishing and emissions of hazardous substances.

Recommendations
- Increase efforts to meet the carbon emission targets agreed at global and EU level.
- Accelerate action to reduce nutrient inputs from land and thus eutrophication, overfishing and emissions of hazardous substances.
- Promote a national and international ban on the discharge of scrubber washwater into the sea, which can cause severe acidification locally, and encourage the development of alternative fuels.
- Extend the acidification monitoring programmes in both space and time on a resolution that is relevant for species and ecosystems, and combine with biological observations.
- Promote biological research on Baltic species and ecosystems to evaluate their sensitivity to ocean acidification in combination with other local drivers.
It is now well recognised that today’s high greenhouse gas emissions are causing climate change and global warming. Less recognised is what is known as “the other carbon dioxide problem” – ocean acidification.
In the 1980s, acidification of soil, streams and lakes was one of the most high-profile environmental problems. Emissions of sulphur and nitrogen oxides from combustion processes in, for example, motor vehicles, power stations and heating plants gave rise to atmospheric deposition of sulphuric and nitric acid, popularly known as ’acid rain’, which had major impacts on lake and forest ecosystems. However, vastly improved treatment of emissions in the 1980s and 1990s, combined with the liming of lakes and rivers, led to a reduction of this type of acidification in the Baltic Sea region.
In recent years, another type of acidification has gained attention – global ocean acidification caused by massive carbon dioxide emissions. So far, these changes have been less evident in the Baltic Sea than in the open ocean, but in the long term acidification will have an impact here too – posing yet another threat to the marine ecosystem.
Oceans dampen the greenhouse effect – but get acidified
Since the beginning of industrialisation, the amount of carbon dioxide in the atmosphere has increased dramatically. Analyses of air trapped in Antarctic ice show that over the past 800,000 years, levels have varied between about 180 ppm (parts per million) during ice ages and 280 ppm during interglacial periods. However, over the last 200 years, carbon dioxide levels in the atmosphere have increased to the current level of around 420 ppm.
Today, about 40 billion tonnes of carbon dioxide are released into the atmosphere annually as a result of fossil fuel combustion, cement production and land use change. Almost half (45 percent) of the emissions accumulate in the atmosphere, while 30 percent is absorbed by terrestrial ecosystems and 25 percent is absorbed by the oceans. The oceans thus contribute to mitigating the increase in carbon dioxide in the atmosphere, and the greenhouse effect, which is why the oceans were long considered primarily as a carbon sink.
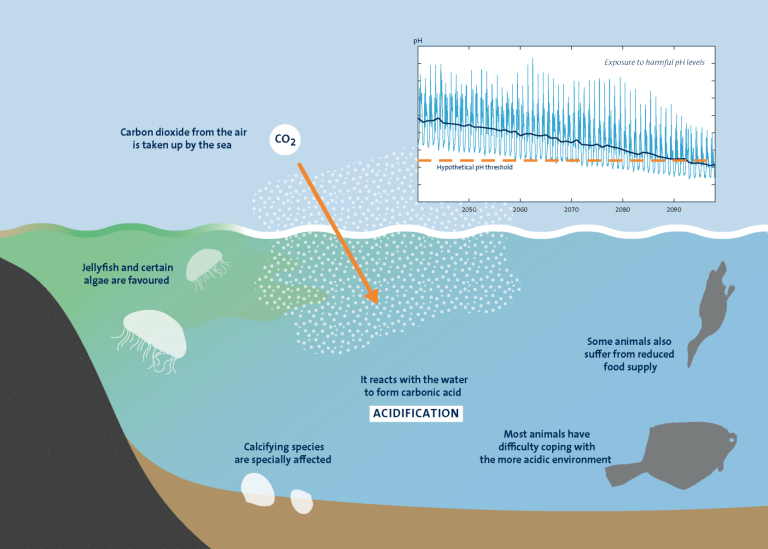
Over time, however, it has become clear that the oceans’ uptake of carbon dioxide is not only positive, but also gradually leads to the acidification of all the world’s waters, including the large oceans. This is because carbon dioxide absorbed by the oceans reacts with the water to form carbonic acid, leading to a gradual decrease in pH.
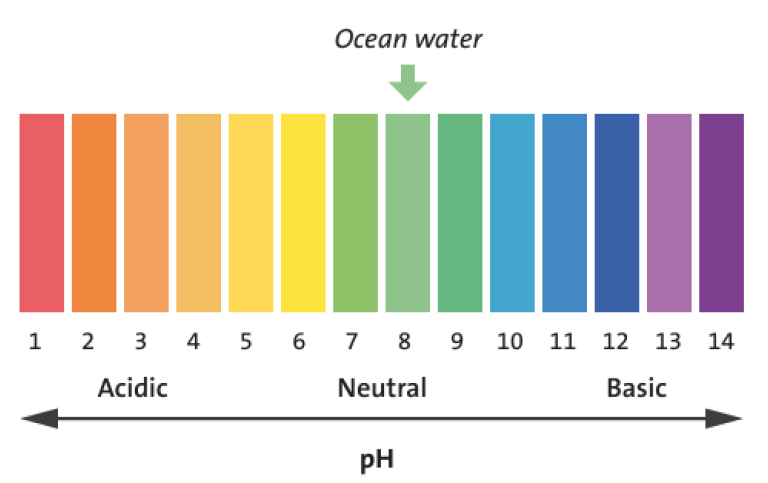
Facts about pH
- pH is used to describe how acidic or basic a liquid is, the pH value is a measure of the concentration of hydrogen ions.
- At room temperature (25°C), pure water is neither acidic nor basic and has a pH value of 7. This is designated as neutral. A lower pH means that a liquid is acidic, and a higher that it is basic.
- Seawater contains, amongst other things, dissolved calcium carbonate and, consequently, is usually slightly basic (pH ≈ 8 on average). However, both geographically and seasonally, there are large variations.
Ocean acidification to date
Currently, pH is decreasing by about 0.002 pH units per year in ocean surface waters. In total, since the beginning of industrialisation, pH has decreased by more than 0.1 pH units to around 8.1 today. This decrease may seem small, but as the pH scale is logarithmic, it represents a change of around 30 percent.
In smaller coastal seas such as the Baltic Sea, long-term pH changes are more difficult to discern because the acidity of the water is simultaneously affected by several other processes that are more significant, and also vary greatly throughout the year.
Geographically, there are large differences in salinity in the Baltic Sea, but pH also differs significantly between the different subbasins and between coastal and open waters. This is partly due to the proportions between ocean water and fresh water in the different basins – the salty and calcareous ocean water usually has a higher pH than fresh water from land. In the south-eastern Baltic Sea, where the bedrock is typically dominated by limestone and sandstone, the river water contains a significantly higher concentration of dissolved calcium carbonate, which makes the water basic, than in the northern parts of the Baltic Sea where the bedrock is largely composed of granite and gneiss. This means, for example, that the water in the Gulf of Riga has a higher pH than that of the Gulf of Bothnia.
Studies show that ocean acidification in some parts of the Baltic Sea, such as the Baltic Proper, so far has been less pronounced than in the world’s oceans. In other parts, pH has decreased faster than in the world’s oceans, such as in some of the Danish bays. Observations show that average annual pH currently is around 8.3 in the Gulf of Riga, 8.1 in the central Gotland Sea and 7.8 in the Gulf of Bothnia.

Expected future acidification
Under current climate policies, it is considered likely that carbon dioxide emissions will peak around the middle of this century and then slowly decline. Such a development would mean that the carbon dioxide concentration in the atmosphere continues to increase to 600 ppm or more by 2100 and that the pH of the oceans’ surface water during the same period decreases by about 0.15 units compared to today’s level. In more extreme climate scenarios that would involve a reversal of some current policies and a sharp increase in carbon dioxide emissions, something that is currently considered less likely, the carbon dioxide concentration in the atmosphere increases to about 1000 ppm by 2100 while pH decreases by 0.3-0.4 units.
Modelling of future development in the Baltic Sea provides a similar picture of how acidification evolves in the longer term – a likely development with current climate policies is an average pH decrease of around 0.1–0.15 units over this century. Although pH levels vary significantly between the different sub-basins of the Baltic Sea, the pH decline over time will be similar. However, in the Gulf of Bothnia the pH decline is expected to be slightly higher than in the central Baltic Sea and in the Gulf of Riga slightly lower.
If the goal of the Paris Agreement – to limit global warming to 1.5 degrees – is realised, the reduction in carbon dioxide emissions would also lead to a halt in global ocean acidification and a slow recovery of pH in the oceans and the Baltic Sea during this century. However, carbon dioxide emissions continue to increase globally and the mitigation measures are currently not on track. Fossil carbon dioxide emissions in 2023 are estimated to be the
highest ever.
Ship emissions can have a large local impact
In addition to global carbon dioxide emissions, emissions of sulphur and nitrogen oxides from vessels can have a significant impact on pH in busy shipping lanes and harbours. New stricter global rules now limit sulphur emissions to the atmosphere. However, one consequence of this has been a sharp increase in the use of so-called scrubber technology.
Scrubber technology is a flue gas cleaning method mainly used to scrub sulphur oxides from ship exhaust gases. The process keeps the sulphur content of the exhaust gases below set limits, but the washwater used and subsequently discharged into the sea is both heavily polluted and highly acidified, often with a pH of around 3. Although the large-scale impact of these emissions on ocean acidification is small compared to that of carbon dioxide emissions, the combination of acidifying and toxic substances in scrubber washwater can have locally significant effects on living organisms.
With more than 10,000 publications in the field of ocean acidification, there is an overwhelming body of evidence showing that for marine ecosystems, the ongoing ocean acidification can have major consequences, favouring some organisms and disadvantaging others.
Most animal species have difficulty coping with the more acidic environment, such as several fish species that appear to be particularly sensitive in the larval stage. Maintaining pH constant in cells and fluids is critical for the good functioning of enzymes and marine organisms do invest a lot of energy in acid-base regulation.
Under ocean acidification, these costs can be significantly increased.
Marine calcifiers, organisms using calcium carbonate to build shells or skeletons, were identified early on as particularly at risk under ocean acidification. This is because of the extra energy costs needed to form and maintain calcified structures.
Impacts on Baltic Sea species
Species living in the Baltic Sea are generally accustomed to and adapted to naturally large variations in pH, and it was first hypothesised that it may be easier for them to cope with ocean acidification than species in the open oceans. However, recent studies showed that organism sensitivity is mostly depending on the extremes in the natural variability in pH, and the annual pH minimum is projected to decrease under ocean acidification. As such, Baltic species may be more at risk than previously anticipated.
Studies on specific Baltic Sea species show that plankton can generally cope with higher carbon dioxide levels in the water, although there are indications that species composition may change. The cyanobacterium Nodularia has been shown in experiments to be disadvantaged by acidification, but may on the other hand be favoured by other effects linked to climate change.
Of the calcifying species, the Baltic tellin (Macoma balthica) has been shown in experiments to have difficulties in coping with acidification. The ocean quahog (Arctica islandica) appears to be more tolerant and adaptable, as is the blue mussel (Mytilus edulis) in the adult life stage. However, in the larval stage, the blue mussel has been shown to be more sensitive and the physiological stress caused by acidification may therefore harm future populations.
For macroalgae such as bladderwrack, acidification may lead to better growth due to the increased availability of carbon which is their energy source, but the effect is likely to be small.
Several Baltic Sea fish species, such as herring and cod, are among the species that are particularly sensitive to acidification in the larval stage, but fish populations can also be affected by changes further down the food web.
Eutrophication amplifies natural pH variations
pH in the sea varies naturally throughout the year due to carbon dioxide being fixed by plants and animals and then released as the organic material decomposes. High inputs of phosphorus and nitrogen often give rise to large algal blooms, which means that large amounts of carbon dioxide are fixed by plants. This also results in a large pH increase during spring and summer when light conditions are favourable for photosynthesis, but also in a large pH decrease med the organic material is decomposed and carbon dioxide is released. During a plankton bloom, pH in the surface waters of the Gotland Sea, for example, can increase by more than 0.5 over the course of a month, and then decrease by the same amount again during the winter, as carbon dioxide is released through decomposition processes.
In a eutrophic sea with high phytoplankton production, the pH increase during the summer is greater than in a nutrient-poor sea, but the decrease during the winter is also more pronounced. Modelling of the development of ocean acidification in the Baltic Sea also shows that mean pH over the year is higher if nutrient supply is at a high level than if it is reduced in accordance with what the Baltic Sea countries have agreed on through the Helcom Baltic Sea Action Plan. At the same time, however, seasonal fluctuations become greater and the annual pH minimum is lower under eutrophic conditions, and that is what have negative impact on marine animals.
Combined effects can hit hard
In parallel with ocean acidification, climate change-related processes are taking place in the Baltic Sea, such as increased water temperature and heat waves, reduced oxygen levels, reduced ice extent, changes in salinity and increased coastal erosion.
In addition, Baltic Sea ecosystems are under stress from overfishing, emissions of hazardous substances, and environmental impacts related to eutrophication, such as large plankton blooms and the proliferation of oxygen-poor deep water areas.
There is a great need for knowledge on how the combination of ocean acidification and other processes will affect the Baltic Sea ecosystem in the future. With future global climate change in mind, there is an urgent need to do what is possible to reduce regional problems in the Baltic Sea, such as eutrophication, overfishing and emissions of hazardous substances. This can make organisms and ecosystems more resilient to future climate and acidification impacts.
Contact
Erik Gustafsson, Stockholm University Baltic Sea Centre
erik.gustafsson@su.se
Monika Winder, Department of Ecology, Environment and Plant Sciences, Stockholm University, monika.winder@su.se
Sam Dupont, Department of Biological & Environmental Sciences, Gothenburg University
sam.dupont@bioenv.gu.se
Read as a layouted pdf
Read or download this policy brief as a layouted pdf:
Ocean acidification poses another threat to the Baltic Sea ecosystem
Read or download a text version with references:
Ocean acidification poses another threat to the Baltic Sea ecosystem
Last updated: February 7, 2024
Source: Baltic Sea Centre

