The mutual neutralization of hydronium and hydroxide
Using the unique DESIREE facility, researchers at Stockholm University and The Hebrew University of Jerusalem have for the first time been able to directly visualise the neutral products of the mutual neutralization of hydronium and hydroxide, and report three different product channels: two channels were attributed to a predominant electron-transfer mechanism, and a smaller channel was associated with proton transfer. The two-beam collision experiment is an important step toward understanding the quantum dynamics of this fundamental reaction. Their findings are published in the journal Science. A team of scientists led by Prof. Daniel Strasser at The Hebrew University in Israel joined with a team led by Dr. Richard Thomas at The Department of Physics at Stockholm University, to investigate this reaction using the DESIREE facility.

The mutual neutralization (MN) of hydronium cation, H3O+, and the hydroxide anion, OH¯ to form neutral water molecules is one of the most basic chemical processes, where the MN by proton transfer (PT) between hydronium and hydroxide ions and the reverse reaction of water autoionization, as this governs the pH of pure water. This process has attracted considerable interest, but direct experimental probing of the underlying reaction mechanisms has been lacking. By realizing the interaction in merged beams of two ionic species with near-zero relative velocity, we were able to directly visualise the neutral products of these reactions and observe three different product channels. Two channels are attributed to a predominant electron-transfer mechanism, and a smaller channel is associated with proton transfer. The two-beam collision experiment is an important step toward understanding the quantum dynamics of this fundamental reaction.
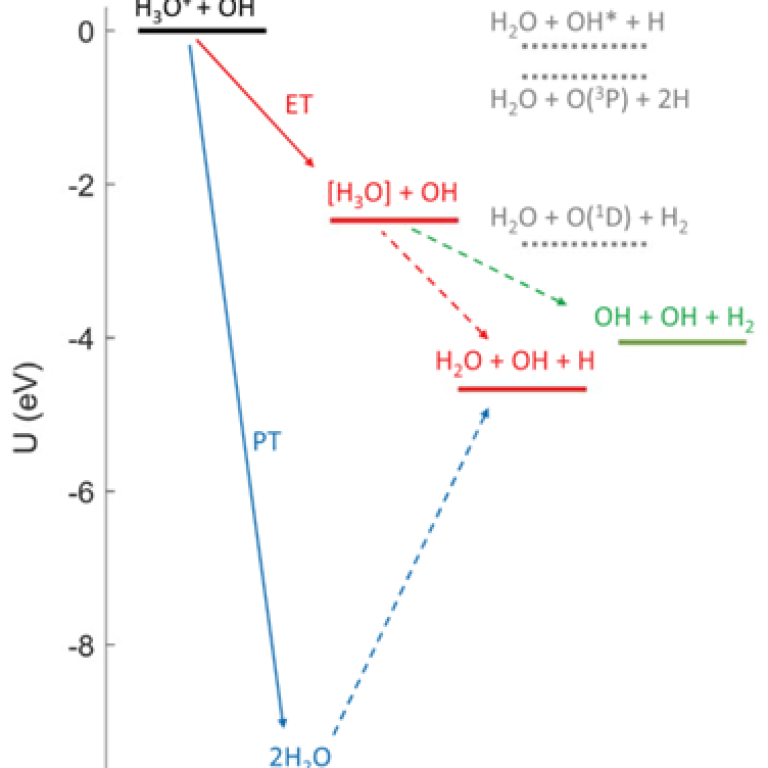
A team of scientists led by Prof. Daniel Strasser at The Hebrew University in Israel joined with a team led by Dr. Richard Thomas at The Department of Physics at Stockholm University, to investigate this reaction using the DESIREE facility. Here, the hydronium and hydroxide ions are created independently, prepared, and allowed to interact in a controlled manner without other nearby molecules interfering. The mutual-neutralization reaction is then measured by coincidence detection of the individual neutral products. In liquid water, proton transfer is the only reaction mechanism, whilst, in the isolated system, electron-transfer dominates and proton-transfer is a minor channel, but could still be identified in DESIREE. “It is exciting to be able to directly observe the competition between the electron and proton transfer mechanisms in this reaction” said Daniel Strasser. The reported mechanism-resolved internal product excitation, as well as collision-energy and initial ion-temperature dependence provide a benchmark for modelling charge-transfer mechanisms in different “water ion” containing environments.
“It’s fantastic that we can take a bottom-up approach to tackling one of physical chemistry’s most difficult challenges,” said Richard Thomas. “We look forward to slowly adding complexity back into the experiment, by adding one water molecule at a time, and studying the effect of this, as, at some point, the electron transfer must decrease such that the proton transfer channel dominates completely, and we’d like to find out when that is”.
“The DESIREE facility was to a large extent motivated by the ability to study mutual neutralization for molecular ions, and this is a milestone for the facility opening a number of possibilities for future studies by DESIREE users” said Prof. Henning Schmidt, director of the DESIREE facility, and co-author on the paper.
Scientists Contact:
Daniel Strasser, strasser@huji.ac.il.
Richard D. Thomas, rdt@fysik.su.se.
DESIREE Facility Contact: Henning T. Schmidt, schmidt@fysik.su.se, Richard D. Thomas, rdt@fysik.su.se.
Further reading in Science: Alon Bogot et al., The mutual neutralization of hydronium and hydroxide. Science383,285-289(2024).DOI:10.1126/science.adk1950
More information
The mutual neutralization of hydronium and hydroxide, Science383,285-289(2024).DOI:10.1126/science.adk1950
Research Group DESIREE at the Department of Physics, Stockholm University
Last updated: January 22, 2024
Source: Fysikum



