A ribbon of resilience: How nanotechnology could reinvent materials for extreme environments
In a study recently published in Science, an international team of researchers from Stockholm University, the University of Illinois Chicago, and several collaborating institutions has unveiled a new class of materials that could revolutionize technologies operating under extreme conditions–from the heat of a jet engine to the crushing pressure deep underground.
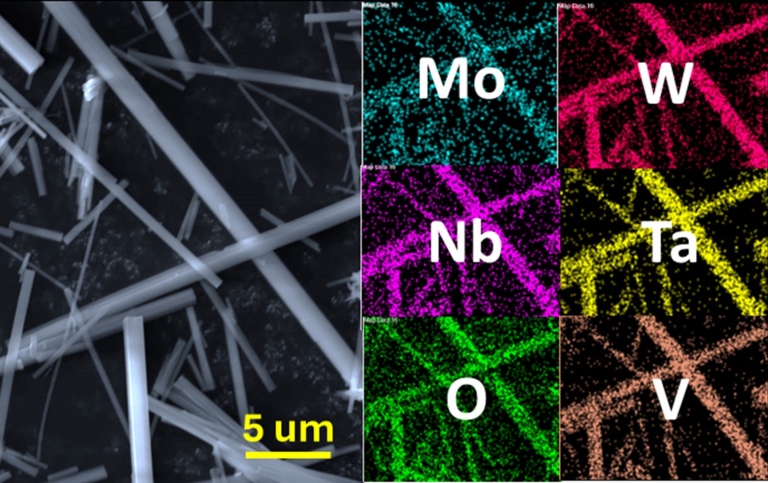
The new material the researchers have found is a one-dimensional high-entropy oxide nanoribbon (1D-HEO), composed of a complex mix of five metal elements: molybdenum, tungsten, niobium, tantalum, and vanadium, bonded with oxygen. This isn’t your average ceramic or alloy–it’s a high-entropy material, designed by mixing many elements in near-equal ratios to harness structural disorder as a stabilizing force rather than a flaw.
Shock-resistant coatings or space missions
What makes this material stand out isn’t just its composition, but its shape and structure, Zhehao Huang, explains. He is the leading researcher of this work at the Department of Chemistry, Stockholm University.
The nanoribbons, which are thousands of times thinner than a human hair, display exceptional resistance to heat (up to 1 000°C), pressure (up to 30 gigapascals), and chemical corrosion (surviving strong acids and bases for a week). Perhaps most impressively, they can absorb mechanical energy better than any currently known aerospace alloy, making them ideal candidates for applications like shock-resistant coatings or structural materials for space missions.
“Cutting-edge electron microscopy techniques”
“Behind our results lie cutting-edge electron microscopy techniques, particularly Transmission Electron Microscopy, TEM, and Three-Dimensional Electron Diffraction, 3DED, which have become indispensable tools in modern materials science”, Zhehao Huang says.
TEM provided ultra-high-resolution images of the nanoribbons, confirming their smooth, defect-free surfaces and uniform internal structure. These atomic-level visuals were essential in verifying that the material maintained its integrity even after exposure to extreme temperatures and corrosive environments.
Three-dimensional atomic structure of nanoribbons
Even more pivotal was the use of 3DED, which enabled the reconstruction of the full three-dimensional atomic structure of these nanoribbons.
“With 3DED, we can determine the atomic arrangement from crystals that are only tens of nanometers in size–something traditional X-ray diffraction simply can’t handle,” Zhehao Huang explains.
This method revealed how metal and oxygen atoms are precisely arranged in the structure.
“It allowed us to detect subtle features like oxygen vacancies and the presence of mixed metal coordination environments, which are crucial for understanding the material’s remarkable stability under extreme conditions.”
These insights were not just confirmatory–they were foundational. Without them, the extraordinary resilience of the nanoribbons would have remained a mystery, Zhehao Huang adds.
Possible to design resilient materials
From electronics that can survive Venus-like heat to spacecraft materials that endure both vacuum and vibration, the potential applications of these findings are vast, the researchers say. What’s more, the study offers a blueprint for designing low-dimensional, resilient materials by harnessing entropy as a stabilizing feature rather than treating it as a challenge.
“Beyond the material itself, the research sets a benchmark for how nanostructures are studied. By combining TEM and 3DED, scientists can now uncover the atomic details of materials that are too small or too complex for traditional techniques. It’s a powerful reminder that in science, how we observe can be just as critical as what we observe,” Zhehao Huang says.
Last updated: June 3, 2025
Source: Communications Office


