Pål StenmarkProfessor of Biochemistry
Research
Structural and biophysical studies of Botulinum neurotoxins and novel Cancer targets in nucleotide metabolism
- The botulinum neurotoxins
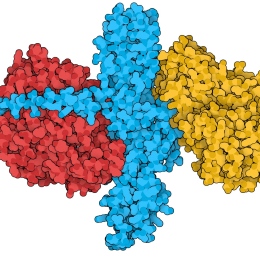
The botulinum neurotoxins are the most toxic substances known; they are one million times more toxic than the cobra toxin. In spite of their extreme toxicity there has been a rapid expansion of the medical applications for the botulinum neurotoxins, with new applications constantly being discovered. This line of treatment is becoming the standard procedure for many conditions. The toxins are possible agents for bioterrorism and the development of countermeasures and vaccines are of high priority. We are studying the toxins using a wide variety of methods, including X-ray crystallography, to understand toxins basic mechanism and to improve their therapeutic properties.
Structure of Botulinum neurotoxin type B bound to two receptors on the neuronal
- Structure based drug design
We also study enzymes involved in the nucleotide metabolism. One example is MTH1, this protein is important for clearing oxidative damage from the nucleotide pool. Many types of cancer cells have high levels of oxidative damage and depend on MTH1 for survival. We use X-ray crystallography to study the binding of MTH1 to natural substrates and potent inhibitors that we are developing in an interdisciplinary research collaboration. We use structural based drug design to refine these inhibitors into novel cancer drugs.
The crystal structure of human MTH1 in complex with a key inhibitor.
Selected Publications
Krč A, Persson Košenina S, Nowakowska M, Masuyer G, Stenmark P. (2025)
Structure of the Complete 14-subunit Botulinum Neurotoxin B Complex Reveals a Unique Anchoring Through the Narrow Central Pore of HA70
Science Advances. 2025 Aug 29;11(35):eadx5058.
Dong M, Masuyer G, Stenmark P. (2019)
Botulinum and Tetanus neurotoxins
Annual Review of Biochemistry. 2019
Zhang S, Masuyer G, Zhang J, Shen Y, Lundin D, Henriksson L, Miyashita S, Martínez-Carranza M, Dong M, Stenmark P. (2017)
Identification and characterization of a novel botulinum neurotoxin
Nature Communications. 2017 Aug 3;8:14130.
Masuyer G, Conrad J, Stenmark P. (2017)
The structure of the tetanus toxin reveals pH-mediated domain dynamics.
EMBO Reports. 2017 Aug;18(8):1306-1317.
Carter M, Jemth AS, Hagenkort A, Page BD, Gustafsson R, Griese JJ, Gad H, Valerie NC, Desroses M, Boström J, Warpman Berglund U, Helleday T, Stenmark P. (2015)
Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2.
Nature Communications. 2015 Aug 4;6:7871.
Gad H, Koolmeister T, Jemth A, Eshtad S, Jacques S, Ström C, Svensson L, Schultz N, Lundbäck T, Einarsdottir, Saleh A, Göktürk C, Baranczewski P, Svensson R, Berntsson R, Gustafsson R, Strömberg K, Sanjiv K, Jacques-Cordonnier M, Desroses M, Gustavsson A, Olofsson R, Johansson F, Homan E, Loseva O, Bräutigam L, Johansson L, Höglund A, Hagenkort A, Pham T, Altun M, Gaugaz F, Vikingsson S, Evers B, Henriksson M, Vallin K, Wallner O, Hammarström L, Wiita E, Almlöf E, Kaldéren C, Axelsson H, Puigvert J, Häggblad M, Jeppsson F, Martens U, Lundin C, Lundgren B, Granelli I, Jenmalm-Jensen A, Artursson P, Nilsson J, Stenmark P, Scobie M, Warpman-Berglund U, Helleday T. (2014)
MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool.
Nature. 2014 Apr 10;508(7495):215-21.
Berntsson RP, Peng L, Dong M, Stenmark P. (2013)
Structure of dual receptor binding to botulinum neurotoxin B.
Nature communications, 2013 Jun 28;4:2058
Funding Sources
Our research is supported by grants from the Swedish Research Council, Novo Nordisk and the Swedish Cancer Society.
Research projects
Publications
$presentationText