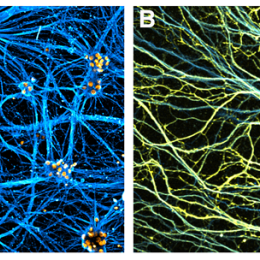
Research group Eva Hedlund's research group
We are focused on understanding mechanisms of selective neuronal vulnerability and resilience in neurodegenerative diseases, with particular emphasis on the lethal motor neuron disease amyotrophic lateral sclerosis (ALS).
Towards this goal we use induced pluripotent stem cells (iPSCs) and organoids generated from these, CRISPR/Casp9 genome editing, microfluidics to create models for in situ analysis with live cell imaging, immunofluorescence, and RNA scope but also single cell RNA sequencing techniques, including the spatial RNA sequencing methods, LCM-seq and Axon-seq that we have developed.
Group description
We investigate cell intrinsic properties of neurons that show either extreme vulnerability or particular resilience and even regenerative properties in response to ALS. The aim is to reveal mechanisms that can be therapeutically targeted to render neurons more resistant to disease.
Towards this purpose, we have developed a highly sensitive spatial RNA sequencing method, LCM-seq, that we use to unravel the response of individual neuron to disease as these are either degenerating, persisting or regenerating in response to ALS. We modulate gene expression in vulnerable neurons, aiming to make them more similar to the resistant ones, and thus increase their survival.
In several neurodegenerative diseases, it is the long neuronal processes (axons) and their synapses (with other neurons or muscle) that first show signs of pathology and degenerate. To reveal early disease processes in axons and identify targets for disease intervention, we have developed a highly robust method (Axon-seq) to sequence the content of axons and to analyze disease-induced dysregulation of the axonal mRNA content. We are now utilizing Axon-seq to increase the understanding of axon biology in general and to unravel early disease mechanisms in ALS using neurons specified from human iPSCs where we have introduced disease-causing mutations using CRISPR-Cas9 genome editing.
We are also building organoids and neuromuscular junctions (NMJs) in vitro from human iPSCs and studying connectivity and communication between motor neurons of different vulnerability and connected cells to unravel how they communicate in health and what goes awry in disease.
Photo: Melanie Leboeuf - Human stem cell derived spinal motor neurons visualised through an Hb9-GFP reporter and staining against Lamin B (red), G3BP1 (white) and Hoechst (blue)
Group members
Group managers
Eva Hedlund
Professor of Neurochemistry

Members
Tina Noreng Karlsson
Student

Maria Francisca Basaran Van Ham
Student

Christina Toumpa
Student

Rianne de Jongh
Guest researcher

Sagar Verma
Postdoc

Larissa Kahnwald
PhD Student

Nils Dennhag
Postdoc

Lara Leal Hernandez
PhD student

Silvia Gómez Alcalde
PhD student

Melanie Leboeuf
Postdoc

Irene Mei
PhD student